Research in mice identifies surprising consequences of a disturbed gut microbiome
“Sometimes, when something bad happens, it leaves a trace of itself behind.” When Scatman Crothers’ character spoke these memorable words in Stanley Kubrick’s film version of Stephen King’s The Shining, he was referring to ghosts. However, there’s increasing evidence that similar considerations apply to the very real world of biology, such that events that befall an organism during its lifetime can have extremely long-lasting consequences, not just for the individual concerned but also for its offspring. Crucially, these effects do not involve change in the DNA sequence of the genome. Instead they are known as epigenetic: changes in the expression of particular genes which occur due to heritable changes in how these genes are regulated that can be passed from fathers or mothers to their children, and potentially even beyond.
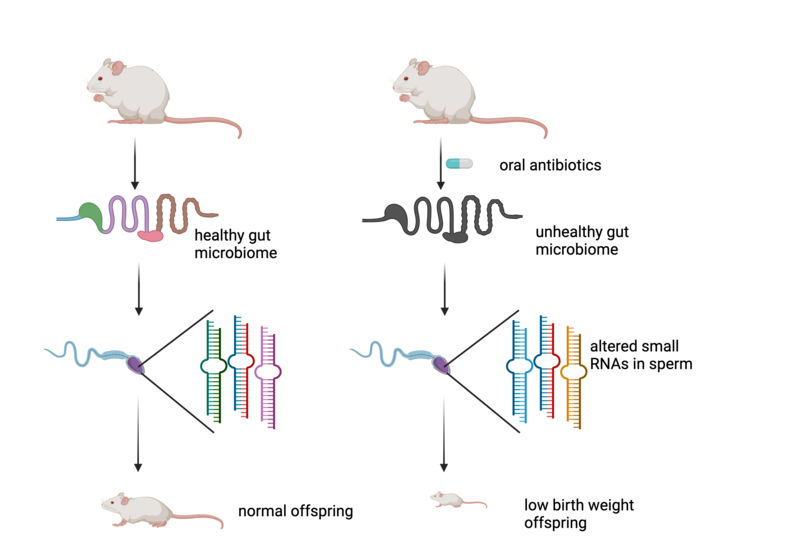
Above: Feeding mice a cocktail of antibiotics results in a severely disturbed microbiome. This leads to changes in the small non-coding RNAs in sperm and low birth weight in the offspring of these mice. Figure created with BioRender
In a new study just published in the journal Nature, Jamie Hackett and his group at EMBL in Rome, aided by the Sarkies lab based here in Biochemistry, discovered a particularly striking example of epigenetic changes transmitted between generations, which could have important implications for humans. The gut microbiome (‘good’ bacteria that live naturally in the gut of animals including humans) is increasingly being recognised as a key aspect of health. Jamie Hackett’s team discovered that disrupting the microbiome by subjecting male mice to a course of 6 weeks of antibiotics, resulted in severely adverse changes in their offspring. In particular, the offspring of antibiotic-treated mice were smaller on average and had a high incidence of infant mortality due to some progeny being born with extremely low weights. So severely disrupting the microbiome has effects not only for an animal, but for its progeny.
What is the molecular mechanism for this phenomenon? The researchers found many changes in the molecules that are present in the sperm of the mice that occurred due to the disturbed microbiome. Amongst these, small non-coding RNA molecules, which regulate genes in cells by recognising specific mRNA and targeting them for destruction, were markedly altered. These changes likely alter the development of the embryo early on, resulting ultimately in reduced weight. Exactly how the microRNAs and other altered molecules might achieve this is still unknown. Crucially though, these are epigenetic effects, which do not involve a change in the DNA sequence but nevertheless affect the offspring of the mice, transmitted through the sperm.
This study shows a clear demonstration of how events that occur to an organism can be transmitted epigenetically into the next generation, and identifies the gut microbiome as a key factor in determining not only an individual’s own health, but that of the next generation too. It will be really important to follow this study up to uncover the exact chain of events whereby the loss of a father’s microbiome leads to defects in embryonic growth of its children. This may further have important implications for antibiotic use, and the health of the microbiome in general, for human reproduction.
Peter Sarkies
30th April 2024




