A collaborative study between Oxford, Glasgow and UKHSA discovers unexpected differences in the replication of SARS-CoV-2 variants of concern
A new study reveals differences in replication rates of SARS-CoV-2 variants of concern using single molecule RNA detection
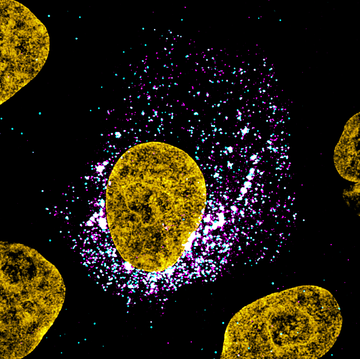
Figure 1: Image of a SARS-CoV-2 infected cell. SARS-CoV-2 genomic RNA smFISH (cyan), viral nucleocapsid protein (magenta), DAPI (yellow). Individual cyan spots represent single molecules of viral RNA. Aggregated signals indicate sites of viral assembly and replication
Research led by Ilan Davis and Jane McKeating labs at the University of Oxford and MRC-University of Glasgow Centre for Virus Research, have quantified SARS-CoV-2 replication at unprecedented detail and sensitivity, providing new insights into transmissibility of variants and susceptibility to infection.
SARS-CoV-2 is responsible for the COVID-19 pandemic. Soon after entry into the host cell, the virus replicates its genetic material so that new viral progeny can be assembled and released to infect other cells. The initial steps of the viral life cycle play an important role in establishing a productive infection, however these early stages of the infection dynamics are still poorly understood.
To address this question, the Davis lab (Biochemistry, University of Oxford), the McKeating lab (NDM, University of Oxford), and Castello lab (MRC University of Glasgow Centre for Virus Research) developed a highly sensitive approach to visualise and quantify the RNA SARS-CoV-2 throughout the infection. By adapting the single molecule fluorescence in situ hybridisation (smFISH) methods established in the Davis lab to work with Drosophila tissues, they were able to detect individual copies of viral RNA genomes in infected cells and lung tissue at greater than 95% sensitivity. Importantly, the authors observed that only a minor population of cells displayed high levels of viral RNA while most cells presented low copies of viral RNA.
“Most of the available techniques to study viral replication rely on studies of cell populations, providing data that is an average between all cells within the culture or tissue. While useful, it does not allow us to see differences between cells or to study the SARS-CoV-2 life cycle with enough detail” – said Prof. Ilan Davis. “Our approach surpasses these ‘in bulk’ techniques because it provides accurate information for each individual cell from the very first time point of infection to the point at which the cell is completely overwhelmed with viral particles. We were also able to show that the technique works in sections from infected lungs of animals, which opens up possibilities in the future to study samples from infected patients”.
“This level of cell heterogeneity is surprising because they were clones. In other words, these cells are in principle near-identical” – said Jeff Lee, one of the first authors of the work.- “I would expect variation between cells in complex tissues, which are heterogenous and contain many different cell types.” “The high variation in SARS-CoV-2 replication in clonal cell lines implies that the cellular state, such as the cell cycle or the response to stress, may define the speed in which the virus progresses – Added Peter Wing, the other joint first author-. It would be of great therapeutic interest to discover the roadmap to understand the determinants of cell susceptibility to infection.”
The study also revealed that SARS-CoV-2 RNAs are long-lived within infected cells. “When we treated the cells with remdesivir, the only known drug, at the time, against the virus, to inhibit viral replication, we observed that the viral RNA persisted for a long time, being detectable for many hours after the treatment – Said Prof. Jane McKeating -. “This could explain why SARS-CoV-2 RNA can be detected in some patients long after the clinical symptoms have resolved.”
Interestingly, this study showed that the alpha variant, detected months ago in the UK, has a slower replication kinetics than the original virus, suggesting that SARS-CoV-2 variants of concern might not only differ in their ability to enter the cells, but also in the speed in which they multiply their RNA. “Most of the attention has been centred on the mutations detected in the virus spike protein they can influence the ability of the virus to enter the cell or to escape vaccine induced or natural antibody responses” – Said Dr. Alfredo Castello -. “However, we should not ignore that there are many other mutations occurring in other viral proteins that can influence virus growth and transmission”.
Finally, the authors were able to demonstrate sensitive detection of virus mRNA in lung tissue sections of infected animals provided by UKHSA. The ability to study viral infection with such sensitive and high-resolution methods in tissue sections, opens the door for future clinical studies of COVID-19. In summary, the authors quantified SARS-CoV-2 infection kinetics with absolute sensitivity using a powerful tool to visualise the sub-cellular localisation of viral RNA. The findings of this paper will be relevant to broader audience of both scientists and clinicians to shed light into the replication dynamics of the current and future variant viruses and how these may affect clinical outcomes.
The paper ‘Absolute quantitation of individual SARS-CoV-2 RNA molecules provides a new paradigm for infection dynamics and variant differences’ is published in eLife. The work was co-led by the Davis lab (Biochemistry, Oxford), as a multi-site close collaboration with scientists in Jane McKeating’s lab (Nuffield Department of Medicine, Oxford), Alfredo Castello’s lab (MRC Centre for Virus Research, Glasgow. The authors would like to acknowledge the help received from Dunn School of Pathology, Oxford, which permitted the setup of a dedicated research lab for the purpose of conducting this work. The authors also thank Olympus Life Sciences for their generosity in loaning the SpinSR10 microscope for this project as well as Jordan Raff and Matthew Freeman for their support.
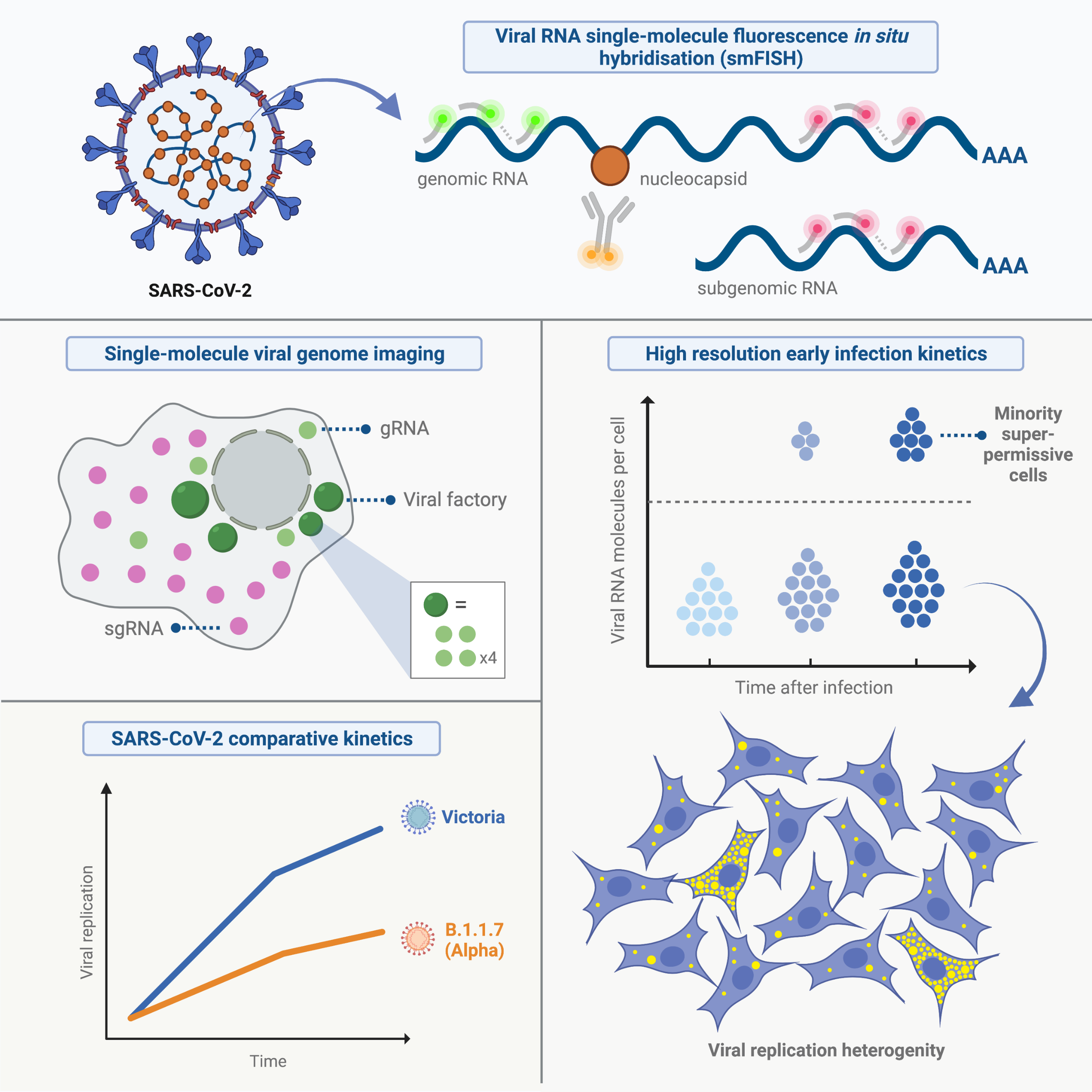
Figure 2: Graphical abstract highlighting major findings of the study
Research groups
Ilan Davis (Biochemistry, Oxford)
Jane McKeating (Nuffield Department of Medicine, Oxford)
Alfredo Castello (MRC Centre for Virus Research, Glasgow)
Francisco J Salguero (UK Health Security Agency, Porton Down)
Daniel Agranoff (University Hospitals Brighton NHS Foundation Trust)
Leading group members
Jeff Lee and Dalia Galia, Davis lab.
Peter Wing, McKeating lab
Marko Noerenberg, Castello lab.
Reference(s)
- Link to eLife article 2022: https://elifesciences.org/articles/74153
- Link to our social media video aimed at layperson: https://twitter.com/ilandavislab/status/1487396079142416389?s=20&t=_SaUmG1OlINd2WwBg9DZcA
Jeff Lee and Ilan Davis
28th February 2022




