The benefits of therapeutic hypothermia have been recognised since ancient times, while today controlled cooling is an integral part of modern medicine. These approaches exploit the reduced metabolic needs in cells at low temperatures to minimise ischemic damage when blood supply to vital organs is interrupted, for example during a number of surgical procedures and transplant organ preservation. Despite the importance of clinical hypothermia, we know very little about the beneficial and detrimental molecular pathways that are activated at such cold temperatures.
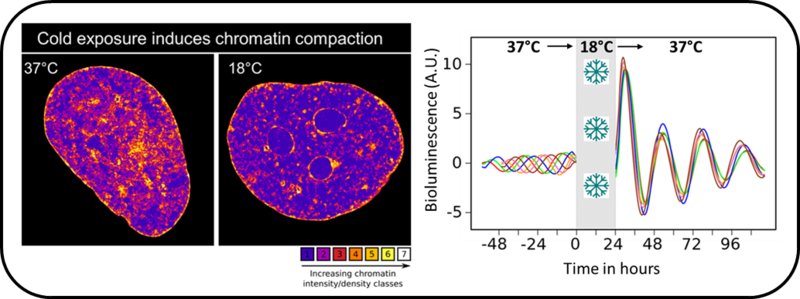
Exposing cardiomyocytes to 18°C compacts chromatin (left) of cells and after rewarming synchronises their circadian rhythms (right). The different coloured lines represent circadian rhythms of cells oscillating at phases that are four hours apart at 37°C
To address this gap in knowledge, Harry Fischl used a multipronged approach, collaborating with the Schermelleh and Mellor labs in the Department of Biochemistry and Aarti Jagannath in the Sir William Dunn School of Pathology, to identify the cold-induced changes in human cardiomyocytes exposed to 28°C, 18°C and 8°C, three temperatures similar to those used in clinical settings. Surprisingly, cellular adaptations were highly temperature-specific with exposure to 18°C inducing the most dramatic changes. At 18°C, chromatin undergoes severe compaction and becomes concentrated at the nuclear periphery, impeding the approach of mRNAs to the nuclear pores. Cells also upregulate the level of more than 1000 gene transcripts, including those of the negative arm of the cellular circadian clock, and these transcripts, due to restricted export, accumulate within the nucleus.
Critically, we could show that these changes were reversible upon rewarming cells back to 37°C, which relaxed chromatin and restored export of the nuclear-accumulated mRNAs, causing a cytoplasmic surge of negative arm clock messages that resulted in resetting of the cellular clock.
Mechanisms that reset the cellular clock are of physiological significance as this clock aligns cellular function with daily environmental cycles by generating rhythmicity in a large proportion of the cellular transcriptome and metabolome.
This work uncovered novel principles of the mammalian cold shock response and identified severe hypothermia-induced nuclear retention of negative clock regulators as a pathway that can synchronise the circadian rhythm in cells.
Reference