New research by the Newstead group, published in Nature, reveals the first crystal structure for a member of the nucleotide sugar transporter (NST) superfamily and provides fundamental new insights into how glycosylation is regulated in the cell.
In eukaryotes, glycosylation occurs in the lumen of the endoplasmic reticulum and the Golgi apparatus. Nucleotide sugars required for glycosylation are imported into the lumen of these organelles by a family of intracellular NSTs. But how NSTs recognize and transport nucleotide sugars has been unclear.
Now, work from the Newstead lab, has revealed the crystal structure of the yeast GDP-mannose transporter, Vrg4. The structure shows how the monosaccharide, GDP-mannose is recognised by the transporter and identifies new sequence motifs responsible for selecting different types of nucleotide sugar molecules in the cell. Intriguingly, the work also reveals that Vrg4 will only function in the presence of short chain lipid molecules, providing the first experimental evidence that membrane bilayer thickness may regulate intracellular transport in the secretory pathway.
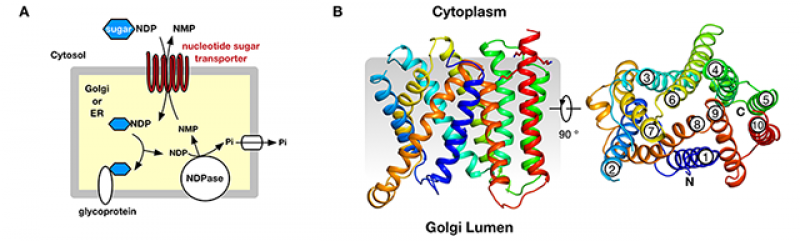
A. Nucleotide sugar transporters function to shuttle activated sugar donors (sugar-NDP) across the endoplasmic reticulum (ER) and Golgi membranes. NDP, nucleoside diphosphate; NMP, nucleoside monophosphate. B, Crystal structure of Vrg4 viewed from the Golgi membrane
The work funded by the Wellcome Trust, paves the way for a molecular understanding of several developmental and immune disorders in humans that are caused by defective glycosylation and provides new opportunities to target pathogenic microbes that exploit the host glycosylation pathway for their virulence.
Determining the final crystal structure was not straightforward, and required long wavelength data collection at the Photon Factory in Japan on several occasions before success was finally achieved.
While characterising the function and properties of Vrg4, an unusual discovery was made. It was found that Vrg4 can only function in the presence of short-chain lipids. The membrane bilayer of the Endoplasmic Reticulum and Golgi Apparatus are thinner than the plasma membrane, and it has been long been speculated that bilayers of different thickness are used as a regulatory mechanism to control aspects of the secretory pathway. The present study demonstrates that this hypothesis is likely to be correct for members of the NSTs and may reflect a mechanism to ensure that NSTs are only active in the correct cellular compartments.
Several pathogenic microbes, including trypanosomatid parasites and fungi, such as Candida albicans, rely of the function GDP-Mannose transporters to glycosylate their surface proteins, a key component of their immune evasion armoury. The crystal structure of Vrg4 reveals that specific pockets can be targeted for inhibitor design, opening up new leads in ongoing drug discovery programmes against these pathogens.
Simon Newstead and Deepa Nath
27th November 2017