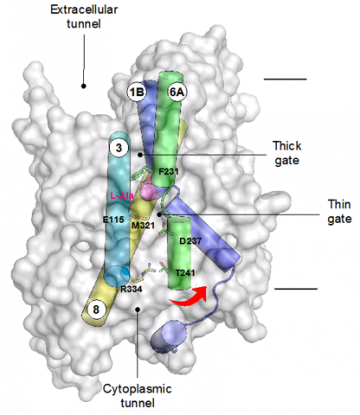
Structure of GkApcT with the predicted gating helices shown. Movement of TM6 away from TM3 and 8 opens the binding site to the interior of the cell, and facilitates release of the bound amino acid
New research by the Newstead group, published in Nature Communications, reveals the first crystal structure for a bacterial homologue of the mammalian cationic amino acid transporters GkApcT providing insight into amino acid selectivity and transport mechanism in this physiologically important transporter family.
Arginine is a major signalling molecule inside the cell, being a precursor for both L-ornithine and nitric oxide (NO) synthesis and a key regulator of the mTORC1 pathway. In mammals, cellular arginine availability is determined by members of the solute carrier (SLC) 7 family of cationic amino acid transporters (CATs), which are found on the cell surface.
Until now the molecular basis of amino acid selection and transport in the SLC7 family has remained unknown, in particular how specificity for cationic amino acids is achieved. In this latest work the group reports the high-resolution crystal structure for a bacterial homologue of the human CATs and identify a single amino acid side chain responsible for regulating affinity for cationic amino acids.
To regulate the uptake of arginine in different tissue and cell types the CAT-2 transporter is also found in two isoforms, CAT-2A and -2B, which differ in their affinity for arginine. Whereas CAT-2B, which is expressed in macrophages and T-cells, exhibits high affinity (mM) for arginine, CAT-2A, which is expressed in liver and muscle cells, displays low affinity (mM). The new structure, combined with biochemical assays have also identified the molecular basis behind this difference, providing new insights into how cellular transport is regulated in eukaryotic cells.
Several inherited disorders are linked to mutations in members of the SLC7 family, including cystinuria and lysinuric protein intolerance (LPI), both of which lead to abnormal amino acid transport, growth defects and kidney disease. Several prescription drugs, including L-DOPA and gabapentin are also recognised by members of the SLC7 family, which are expressed in the blood brain barrier and influence the transport and retention of drug molecules into the central nervous system. The new structure and assays will now facilitate further studies on the mammalian SLC7 transporters to identify the molecular basis for drug transport and the role of identified mutations in disease progression.
Simon Newstead and Deepa Nath
15th February 2018