Mediating gene activation during development
New Research by the Klose Group
An important study; the focus of the lab over the past 4 years
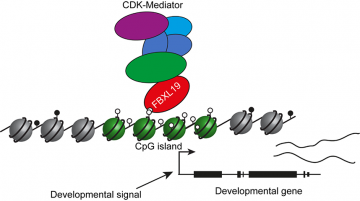
Figure 1. FBXL19 recruits Mediator to CpG islands of developmental genes, priming them for activation upon lineage commitment
The human body is an immensely complex biological machine composed of diverse cells, tissues, and organs. Remarkably, this diversity originates from one single cell, the fertilised egg. This contains all the information, encoded in our DNA as genes, necessary for the development of a fully functional organism. At the heart of forming this diverse complement of cell types is the ability to identify and use a subset of this information at the right time and place during development. When genes are not used in just the right way and at just the right time, normal development is perturbed leading to human disease. Therefore, a central question in human biology is to understand how the usage of genes is controlled in a timely and precise manner during development. This is a prerequisite for understanding how these processes go wrong in disease.
The Klose lab focuses on this important question through understanding the detailed molecular processes that underpin accurate gene usage. In new work published in the journal eLIFE, the Klose lab have uncovered a previously unknown molecular mechanism that controls how certain genes are turned on as cells undergo specialisation, or lineage commitment, during development. In particular, the group discovered that a protein, called FBXL19, physically interacts with a large protein assembly called the Mediator complex. Mediator has been previously shown to play important roles in turning genes on during development, but how it selected the subset of genes that it activates has remained poorly defined.
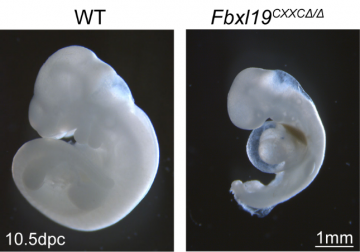
Figure 2: FBXL19 is essential for embryonic development
Through studying the biochemistry of FBXL19 inside the cell, the Klose lab show that FBXL19 can recognize where genes are in the genome, by using a feature called a ZF-CXXC domain to identify DNA sequences called CpG islands. Therefore, FBXL19 effectively tells Mediator which genes it should prepare, or prime, for activation when the right developmental signals are present (Fig 1). The Klose lab discovered this through using stem cells isolated from mouse embryos and genetic engineering to disrupt FBXL19. By growing these cells in a petri dish and inducing them to undergo specialisation, they showed that cells lacking FBXL19 could not turn genes on appropriately during the process of lineage commitment. Importantly, when FBXL19 function was disrupted in mouse embryos, they failed to develop normally resulting in embryonic lethality (Fig 2). Together these discoveries reveal that genes must be recognised and prepared by FBXL19 and Mediator to be activated during specialisation, providing a new molecular rationale for how gene regulation is controlled during development.
This important study, a focus of the Klose lab over the past 4 years, was a highly collaborative and international team effort. Work in Oxford was spearheaded in the Klose lab by Dr Emilia Dimitrova with computational expertise contributed by Dr Angelika Feldmann and important contributions from Dr Benedikt Kessler's proteomics group. The coupling of biochemistry, genomics and developmental biology in this unique interdisciplinary study was made possible through a close collaboration with Dr Haruhiko Koseki's group and Dr Takashi Kondo from the RIKEN institute in Japan. This work was funded by the Wellcome Trust, European Research Council, and The Lister Institute of Preventive Medicine.
Reference
- Dimitrova et al., eLIFE 2018 DOI: 10.7554/eLife.37084
Rob Klose
5th June 2018




