The parasites that cause malaria hide within our red blood cells, multiplying and causing disease. Meanwhile, our immune systems try to eliminate them. Amongst the key weapons available to us are our natural killer cells, which recognise and destroy infectious agents. At the same time, these must leave the cells of our own bodies unscathed. How do malaria parasites avoid being detected and cleared?
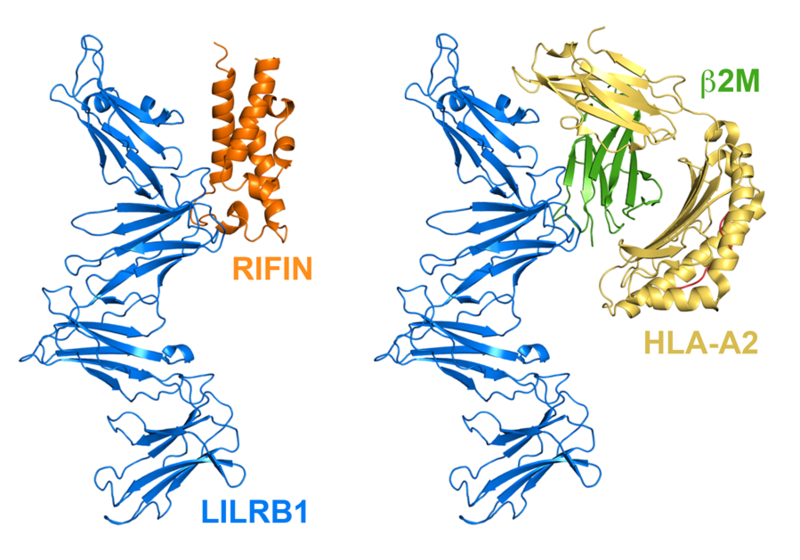
Left: RIFIN bound to LILRB1 Right: MHC bound to LILRB1
The malaria parasite places proteins, called RIFINs, on infected red blood cell surfaces. This is surprising, as we would expect this to make detection of the parasite by our immune system more likely. So, what do RIFINs, and related molecules, do?
Studies by Hisashi Arase’s laboratory identified a group of RIFINs which bind to the inhibitory immune receptor, LILRB1. This receptor is found on human immune cells, such as natural killer cells. Its role is to recognise our own cells and signal to the killer cells to leave them undamaged. To do this, LILRB1 recognises the MHC molecules which mark the surfaces of many of our healthy cells.
Tom Harrison set out to understand what effect RIFINs have on LILRB1. He showed, using structural biology, that the RIFINs mimics MHC molecules in the way that they bind to LILRB1. The malaria parasite therefore directs the natural killers not to destroy infected red blood cells and not to clear the parasites inside.
Working with Alex Mørch, James Felch and Mike Dustin at the Kennedy Institute, we confirmed that this molecular mimicry allowed effective signalling to natural killer cells. Normally these cells become activated and deposit a pore-forming toxin on the surface of a pathogen, but they do not deposit perforin on healthy human cells. The presence of the RIFIN blocked perforin deposition.
Finally, working with Akihito Sakoguchi and Hisashi Arase from Osaka, and Adam Reid from the Sanger Institute, Tom showed that other RIFINs interact with LILRB1 using the same mode of binding.
This study shows that the parasite that causes the most-deadly form of human malaria pretends to be human. It produces a molecule that mimics a marker of healthy human cells, telling our immune cells to leave it unscathed. This cunning piece of molecular mimicry helps the malaria parasite to hide within our bodies, allowing it to multiply and cause disease.
With many more RIFINs available for deployment by the parasite, we expect this parasite protein family to yield many more fascinating stories of human immune manipulation.
Reference
- Harrison, T.E., Mørch, A.M., Felce, J.H., Sakoguchi, A., Reid, A.J., Arase, H., Dustin, M.L. and Higgins, M.K. (2020) Structural basis for RIFIN-mediated activation of LILRB1 in malaria. Nature https://doi.org/10.1038/s41586-020-2530-3
Matthew Higgins
13th July 2020