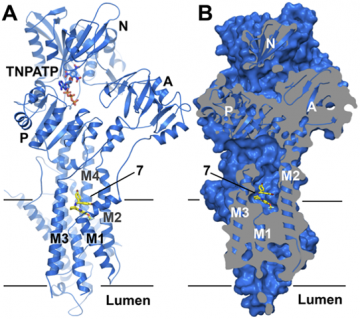
A) Cartoon representation of the Ca2+-pump bound to a HyC (labeled 7). Pump domains and ligands are indicated. The bound HyC and ATP analogue TNPATP are shown as sticks (C: yellow or marine, O: red, Br: dark red, F: pale cyan, N: blue, P: orange) B) Sliced surface view revealing the the ligand-binding pocket at the membrane interface
New research by the Bublitz group in collaboration with the Danish drug discovery company PCovery, describes a new class of compounds that inhibit the growth of fungal cells by disrupting their plasma membrane potential.
Fungal infections cause millions of deaths worldwide and safer, broad-spectrum drugs are urgently needed. In fungi, protons are pumped across the cellular plasma membranes to build up an electrochemical potential that is used to import essential nutrients, such as glucose and amino acids. The potential is built up by a large and complicated enzyme, an ATP-driven proton pump. This pump is conserved in all fungi and is essential for their survival. Notably, as there is no counterpart of this enzyme in humans, researchers at PCovery have been screening for compounds that would shut it down and thereby kill infectious fungi.
In a new research article published in PLOS ONE, work from the Bublitz lab and PCovery, has revealed a new class of small molecule compounds, known as tetrahydrocarbazoles (HyCs), that are potent inhibitors of the fungal proton pump. These compounds cause a breakdown of the membrane potential in fungal cells and inhibit the growth of fungi in laboratory cultures. In order to understand, how HyCs act on the proton pump, the researchers needed a three-dimensional molecular model of the pump bound to one of the compounds. However, as it was not straightforward to obtain high resolution structural information on the fungal proton pump, Bublitz and co-workers took advantage of an otherwise undesired side effect: the set of HyCs they used were not specific to the fungal proton pump but also inhibited a related mammalian Ca2+-pump. By exploiting this cross-reactivity, they succeeded in determining a crystal structure of the Ca2+-pump bound to a HyC.
The structure reveals that the HyC binds in a deep pocket in the pump's surface, in a site where the transported ions are thought to enter, suggesting that the compound causes a blockage of the ion pathway. With this structural information, it was possible to build a molecular model of the fungal proton pump and use it to evaluate the observed effects of different HyCs on proton pumping and fungal growth by an in silico method called molecular docking. The analysis also identified certain sidechains that could be exploited to enhance the selectivity of HyC compounds.
This work paves the way for a further development of the HyC compound series to improve their specificity and efficacy against the fungal proton pump, a crucial step before these compounds can be used as antifungals in clinical trials.
Maike was supported by the Danish Council for Strategic Research and by the Academy of Medical Sciences, UK.
Maike Bublitz and Deepa Nath
3rd January 2018