Understanding cerebral malaria: novel molecular insights into a sticky problem
Why do the most debilitating cases of malaria affect the brain, leading to cerebral disease? This important question has been addressed in a recent paper from Frank Lennartz and Matt Higgins, working with colleagues at the University of Copenhagen [1].
Cerebral malaria is a devastating disease in which erythrocytes infected with the malaria parasite, Plasmodium falciparum accumulate within tiny capillaries of the brain. This restricts blood flow and results in brain inflammation and swelling. It causes life-threatening symptoms and severe long-term neurological damage even in those who survive.
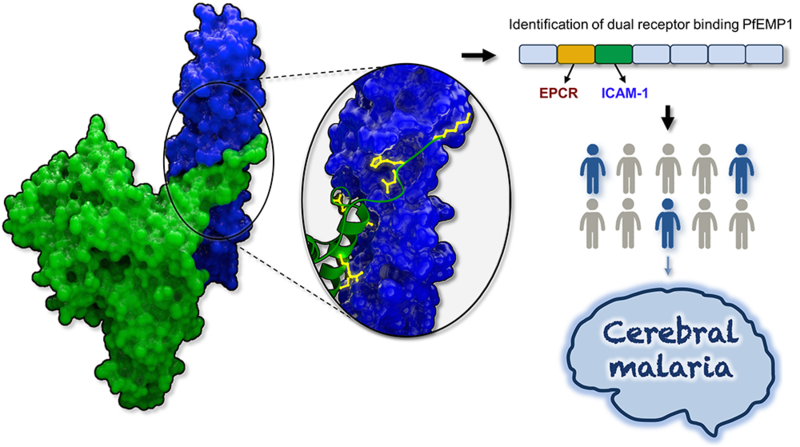
Figure 1. The structure of a complex between a PfEMP1 domain (green) and the human receptor ICAM-1 (blue) was used to determine key residues (yellow) important for receptor binding. This allowed for the identification of dual-receptor binding PfEMP1. The expression of these PfEMP1 correlates with the development of cerebral malaria in children
Infected erythrocytes stick to human tissues via parasite proteins that belong to the PfEMP1 family. These proteins interact with a number of human receptors on endothelial cells, including EPCR, CD36 and ICAM-1. Previous work by the Higgins group revealed the features used by PfEMP1 to bind to both EPCR [2] and CD36 [3] and contributed to a study by colleagues from Copenhagen which identified that PfEMP1 that bind to EPCR are commonly expressed by parasites causing severe childhood malaria [4]. However, a major question remained: which PfEMP1 enable infected erythrocytes to stick in the brain and cause cerebral malaria?
In their new study in Cell Host and Microbe [1], the Higgins group solved a crystal structure of a PfEMP1 protein bound to ICAM-1, revealing the molecular features that allow these proteins to bind each other. Surprisingly, these features are highly conserved, allowing them to define a sequence motif that was then used to search a database of all known PfEMP1 sequences. This identified over 100 PfEMP1 that were previously not known to bind ICAM-1. Intriguingly, all of these PfEMP1 proteins also contained a protein module that allows them to bind to a second receptor, EPCR.
To test whether the ability to simultaneously bind two receptors at the same time has consequences for patients infected with parasites expressing such PfEMP1, the sequence motif was next used to study the PfEMP1 proteins expressed in young African patients with varying malaria symptoms. This revealed a striking correlation between the expression of these dual EPCR- and ICAM-1-binding PfEMP1 and the likelihood of developing of cerebral malaria.
The finding that parasites that produce these dual binding PfEMP1 proteins are more likely to be found in patients with cerebral malaria identifies an important new risk factor for the development of this disease. It also provides a novel avenue for vaccine development, and the remarkable conservation of the ICAM-1 binding site highlights it as a potential vaccine immunogen. With the high mortality rates associated with cerebral malaria, vaccines that prevent binding to the brain blood vessels surfaces, and stop cerebral malaria complications have a strong potential to form part of the urgently needed malaria vaccines of the future.
- Lennartz, F., Adams, Y., Bengtsson, A., Olsen, R.W., Turner, L., Ndam, N.T., Ecklu-Mensah, G., Moussiliou, A., Ofori, M.F., Gamain, B., Lusingu, J.P., Petersen, J.E.V, Wang, C.W., Nunes-Silva, S., Jespersen, J.S., Lau, C.K.W., Theander, T.G., Lavstsen, T., Hviid, L., Higgins, M.K.*, Jensen, A.T.R * (2017) Structure-guided identification of a family of dual receptor binding PfEMP1 that are associated with cerebral malaria. Cell Host and Microbe 21 403-414
- Lau, C.K.Y, Turner, L., Jespersen, J.S., Lowe, E.D., Petersen, B., Wang, C.W., Petersen, J.E.V., Lusingu, J., Theander, T.G., Lavstsen, T. and Higgins, M.K. (2015) Structural conservation despite huge sequence diversity allows EPCR binding by the PfEMP1 family implicated in severe childhood malaria. Cell Host and Microbe 17 118-129
- Hsieh, F., Turner, L., Bolla, J.L., Robinson, C.V., Lavstsen, T. and Higgins, M.K. (2016) The structural basis for CD36 binding by the malaria parasite. Nature Comm 7 12837
- Turner L., Lavstsen T., Berger S.S., Wang C.W., Petersen J.E.V., Avril M., Brazier A.J., Freeth J., Jespersen J.S., Nielsen M.A., Magistrado P., Lusingu J., Smith J.D., Higgins M.K., Theander T.G. (2013) Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 498 502-5.
Deepa Nath
15th March 2017




