Members of the Higgins group have made new discoveries about how malaria parasites get inside our red blood cells which might help them to design new vaccines.
For the most-deadly malaria parasite, Plasmodium falciparum, to replicate within our bodies, it must get inside our red blood cells. At the heart of this invasion process is a large assembly of parasite proteins containing the three components that form the PfRCR complex.
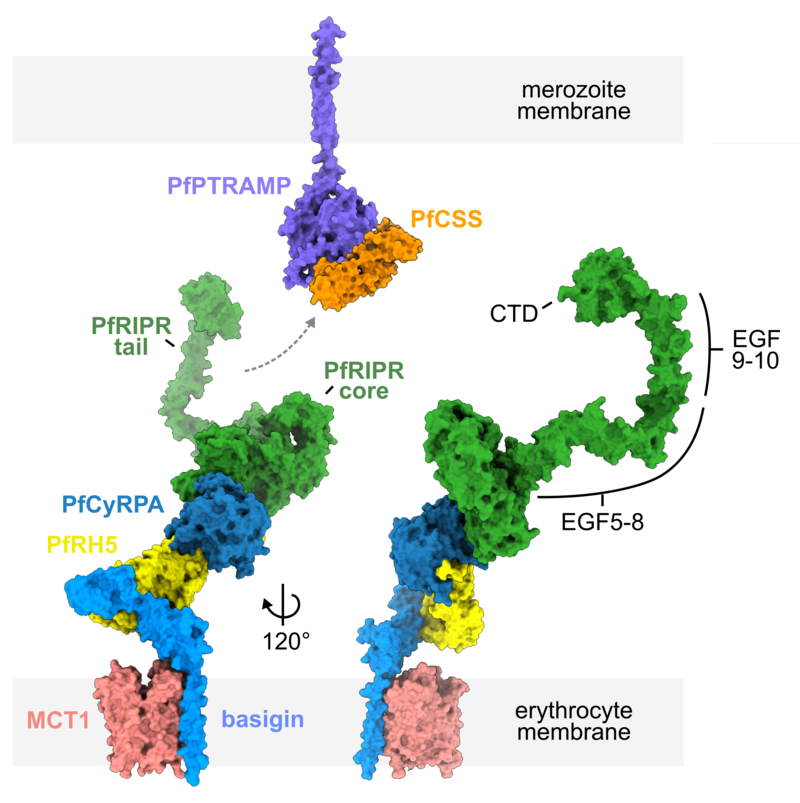
A structural model for how the PfRCR complex bridges the parasite and erythrocyte membranes
Each component of this complex is essential for successful erythrocyte invasion and antibodies against these components can block red blood cell invasion and protect from the symptoms of malaria. Each of these components is therefore a promising vaccine immunogen.
To reveal how these molecules function during invasion, Brendan Farrell set out to determine the structure of the PfRCR complex which contains PfRH5, PfCyRPA and PfRIPR. By using cryo-electron microscopy, Brendan was able to see PfRCR for the first time. This showed that PfRIPR consists of a compact core which binds to PfCyRPA, flexibly attached to an elongated tail.
We also tested the function of PfRH5. A previous study had suggested that PfRH5 might disassemble and insert into the red blood cell membrane during the invasion process. Nawsad Alam therefore locked the two halves of PfRH5 together using disulphide bonds and Melissa Hart and Ellen Knuepfer at the Royal Vet College showed that this did not affect the ability of PfRH5 to mediate cell invasion. Therefore, PfRH5 does not come apart to insert into the membrane during invasion.
Instead, Brendan found that the PfRIPR tail binds to the PfCSS:PfPTRAMP proteins which are found on the parasite membrane. The PfRIPR core assembles with PfCyRPA and PfRH5 to form a unit which binds to the human receptor basigin on the erythrocyte. Therefore, PfRIPR forms a bridge, joining the parasite membrane to the erythrocyte membrane.
This new view of PfRIPR will help us to design and test improved components for a more focused blood stage malaria vaccine. It also provides a better view of what this set of parasite proteins are doing during invasion, opening the way to further experiments to understand how this elusive parasite finds itself a safe home in which to replicate.
Reference: Farrell, B., Alam, N., Hart, M.N., Jamwal, A., Ragotte, R.J., Walters-Morgan, H., Draper, S.J., Knuepfer, E. and Higgins, M.K. (2023) Structure of the PfRCR complex which bridges the malaria parasite and erythrocyte during invasion. Nature doi: 10.1038/s41586-023-06856-1
Matt Higgins
2nd January 2024