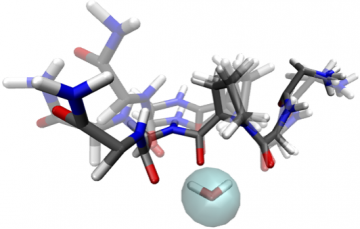
Figure 1 showing the water bridge in KGPGK
The mechanism by which proteins fold from their primary sequence, encoded by DNA, into their specific three dimensional functional structure is still not understood. In nature, this process occurs spontaneously, and relatively quickly, and in water. While there must be an interplay between the specific sequence of the protein and the surrounding water solvent for folding to occur in nature, there is still an ongoing debate concerning how water molecules aid in driving folding. One difficulties in investigating the role of water in this process is because naturally occurring proteins fold rapidly and as a result, it is difficult to observe proteins in the process of folding, especially in the initial stages, by many experimental techniques.
A recent publication in the Journal of the American Chemical Society from research performed in the McLain Group from DPhil student Nicola Steinke, has focused on investigating the roles that both water and the primary amino acid sequence have in initiating β-turn formation in solution By using a combination of techniques which provide information at the atomic level - neutron and X-ray diffraction and computer simulations - the interplay between conformation and hydration for five KGXGK peptides (with X=P,G,S or L) in the process of folding has been determined. These specific peptides were chosen and they will have a distribution of folding states in the solution. As a result, water's role in the folding process can be directly determined.
From this work a consistent two-step mechanism for turn formation in all of the peptides was revealed. First, a water bridge aids in bringing the two ends of the peptide together, forming an intermediate state (see Figure 1 showing the water bridge in KGPGK). This yields an intermediate conformation that puts the β-turn forming motifs in the peptide in close enough proximity to complete the folding structure commonly found in proteins. Whilst the folding mechanism is consistent regardless of the side chain in the X position, only KGPGK shows a highly stabilized intermediate folding state. This may explains the prevalence of the -PG- sequence in -turn sequences observed in the structures of fully folded proteins and may be the site where folding is initiated in many protein structures.
Reference
- Proline and water stabilization of a universal two-step folding mechanism for β-turn formation in solution Steinke, N; Genina, A; Gillams, RJ; Lorenz, CD and McLain, SE Journal of the American Society, DOI: 10.1021/jacs.8b03643 (2018)
Sylvia McLain
1st June 2018