
Sam Gérard
The bacteria Mycobacterium ulcerans causes the horrible disease of Buruli Ulcer. At the heart of its pathology is a secreted molecule called mycolactone. This acts as an inhibitor of the Sec translocon; a universal system which moves proteins across membranes and is essential for protein secretion. By blocking the Sec translocon, mycolactone is thought to prevent secretion of important inflammatory mediators, aiding infection and ulcer formation.
How do inhibitors of the Sec translocon work? A chance meeting with Rachel Simmonds at the WACCBIP conference in Ghana was the start of a collaboration to answer this fascinating question.
Sam Gérard led this project, using cryoelectron microscopy to visualise the ribosome-bound mycolactone-blocked translocon. The structure provided a surprise. The Sec translocon is a highly dynamic molecule with two distinct gates. A plug controls the opening of a channel which passes across the membrane. Meanwhile, a second gate, known as the lateral gate, opens into the membrane environment. Rather than being locked closed, the presence of the inhibitor mycolactone stabilises a partly open state of the translocon, with the cytoplasmic side of the lateral gate opened. This conformation of Sec is poised to be fully opened by the translocating polypeptide.
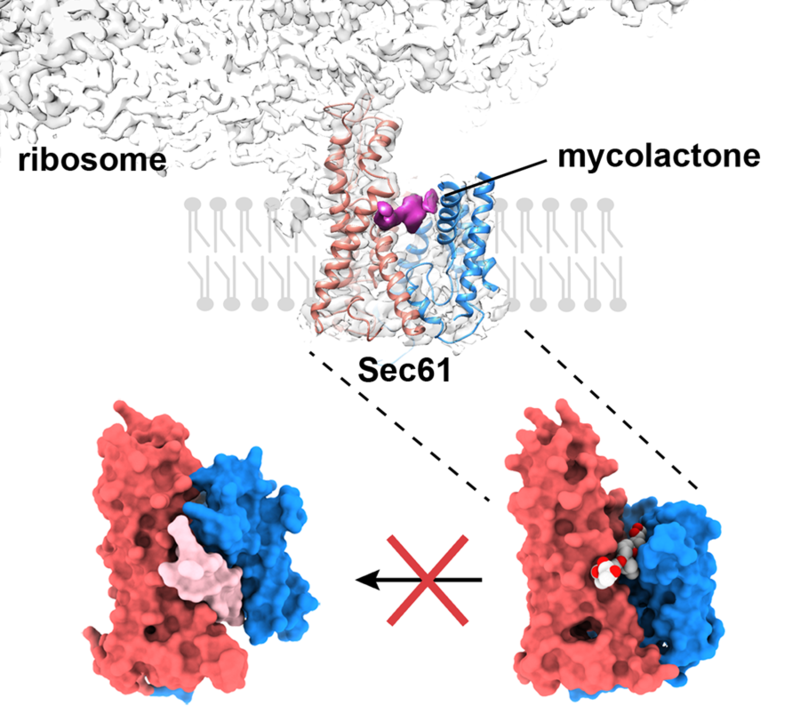
Bottom-left: Active signal-peptide bound Sec translocon. Bottom-right: Mycolactone-inhibited Sec translocon
Mycolactone lies across the entrance to the translocon, occupying a cavity created when the cytoplasmic side of the lateral gate opens. Molecular dynamics work by Afroditi Zaki and Phil Biggin confirmed this, and explored the dynamics of the inhibitor-bound translocon. Mycolactone in this site will block the signal peptide from engaging the translocon and will prevent its full opening.
This study shows, for the first time, the structure of an inhibited conformation of the Sec translocon, providing clues to guide the design of drugs to block this essential piece of cellular machinery.
Reference
- Gérard, S.F., Hall, B.S., Zaki, A.M., Corfield, K.A., Mayerhofer, P.U., Costa, C., Whelligan, D.K., Biggin, P.C., Simmonds, R.E.* and Higgins, M.K.* (2020) Structure of the inhibited state of the Sec translocon. Molecular Cell https://doi.org/10.1016/j.molcel.2020.06.013
Matt Higgins
14th July 2020