The group of Professor Ben Berks reports today in Nature that bacteria that are abundant in the gut and oral cavity assemble their outer membrane proteins using unusually elaborate machinery.
Professor Berks explains: “Most bacteria are enclosed by an outer membrane, which serves as their interface with the environment or host and forms the first line of defence against antibiotics and immune attacks. The proteins found in the outer membrane (OMPs) have distinctive transmembrane β-barrel structures that are inserted into the membrane by the β-barrel assembly machinery (BAM). BAM is essential for cellular function and is a target for certain antibiotics.
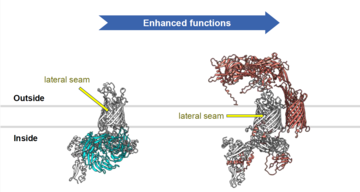
Figure. Comparison of the structure of Bacteroidota BAM with that of the canonical BAM complex of E. coli.*
“A large group of bacteria important for human health, called the Bacteroidota, have unusually elaborate OMPs, often with very large cell surface domains and cell surface subunits that are attached to the membrane through a lipid anchor. These elaborate OMPs enable Bacteroidota species to occupy their niches as beneficial components of the gut microbiome or as pathogens.”
Drs Xiaolong Liu and Luis Orenday Tapia in the Berks group, working with structural biology collaborators Dr Justin Deme and Professor Susan Lea from NIH Frederick and St Jude Children’s Research Hospital, have now isolated and structurally characterized the BAM complex that assembles these unusual Bacteroidota OMPs, and find that it is radically modified relative to BAM in other bacteria.
Compared to the well-studied BAM complex of Escherichia coli Bacteroidota BAM possesses a large canopy structure that extends into the extracellular environment to interact with substrate OMPs as they are folding, and which is essential for Bacteroidota BAM function (Figure). Bacteroidota BAM also contains a protein occupying the substrate-binding site that the researchers show protects Bacteriodota BAM from inhibition by the antibiotic darobactin.
Professor Berks adds: “This work shows that the universally conserved machinery for OMP biogenesis is heavily modified in different bacteria to allow the production of specific types of OMPs. It is also reveals how one group of bacteria have protected themselves from BAM-targeting antimicrobials.”
Read the full Nature article here.
*Figure: Proteins shared by both complexes are coloured white. Proteins found only in the E. coli BAM complex are coloured cyan. Proteins found only in the Bacteroidota BAM complex are coloured red. The assumed position of the outer membrane is indicated by grey lines with the cell exterior at the top of the figure. The lateral seam is the site at which folding of the substrate OMP occurs and the binding stie for the BAM-specific antibiotic darobactin.